Cellular and T cell engager Immunotherapy
Teclistamab in real life : Can we shorten the ramp-up ?
P-049: Teclistamab in real life : Can we shorten the ramp-up ?
Wednesday, September 25, 2024
- JS
Introduction: Teclistamab (TEC) is a bispecific BCMA-CD3 directed T-cell antibody (BsAb) approved from the MajesTEC-1 study with high overall response rates(ORR)≥ 63.0% in refractory/relapsed multiple myeloma (RRMM) patients(pts). The very low rate of ICANS (3.0%; all grade (G) 1 or 2) and CRS (G 3: 0,6% no G 4) was also observed in our center which motivated us to shorten the dose escalation schedule. Thus we report the first results of modified ramp-up of TEC.
Methods: We enrolled all pts with RRMM from a single institution after at least two lines of therapy including triple-class exposure to an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 antibody ; 13 pts had standard ramp-up (Gp1) over 5 days accorded to the guidelines ; 27 pts had received a shortened ramp-up (Gp2) over 4 days (Day(D)1: 0.06mg/kg, D2: 0.3 mg/kg, D4: 1.5 mg/Kg) with no pause between the 1st and the 2nd dose . Data was collected from medical records between september 2022 until 01/05/2024.
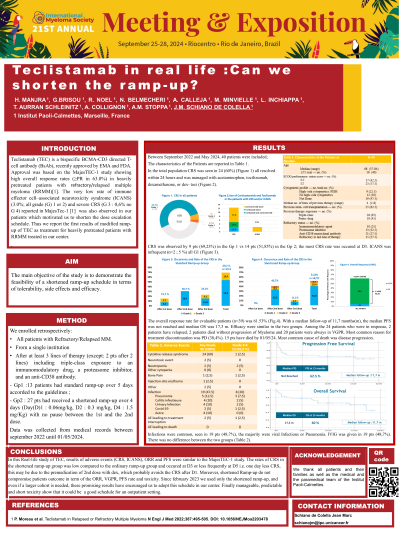
Results: Between September 2022 and May 2024 , 40 patients were included; median age was 68 (range 37-84); 16 pts (40 %) had >75 years; 14 pts (35 %) were males. Patients received a median of 4 of lines of previous therapy (range 2-8); 28,1% were refractory to last line of therapy; 9 pts (22.5%) had high-risk cytogenetics/FISH.
In the total population CRS was seen in 24 (60%) (all G1: except 2 G2, 1 G3), all resolved within 24 hours and was managed with acetaminophen alone (n=17); tocilizumab(toci) alone (n= 1) ; dexamethasone(dex) alone (n=3) or dex- toci (n=2).
CRS was observed by 9 pts (69,23 %) in the Gp 1 (77,8 % G1, 22,2% G2), 22,2 % at D2; 44,4% at D4; 33,3% at D6 vs 14 pts (51,85%) in the Gp 2 (78,5% G1, 14,2% G2, 7,1% G3) 78% at D3, this may be due to the premedication of 2nd dose with dex which probably avoids the CRS after D1; and 21,4% at D5. ICANS was infrequent (n=2 ; 5 %) all G1.
The overall response rate for evaluable patients (n=39) was 61.53%; (≥VGPR in 22; PR in 2; PD in 15). With a median follow-up of 11,7 months(m), the median PFS and OS were 12,14 m and 13,04 m respectively. The median of duration of response (mDOR) is 12,47 months. Efficacy were similar in the two groups. Most common reason for treatment discontinuation was PD (38,4%). 15 pts have died by 01/05/24.Most common cause of death was disease progression.
Infections were common, seen in 19 (48,7%) of pts, the majority were viral Infections or Pneumonia. IVIG was given in 19 pts (48,7%). There was no difference between the two groups.
Conclusions: In this study, results of adverse events (CRS, ICANS), ORR and PFS were similar to MajesTEC-1. The rates of CRS in Gp2 was low compared to the Gp1 and occured at D3 or less frequently at D5 i.e. one day less CRS. Moreover, shortened Ramp-up do not compromise pts outcome and toxicity. Since 02/23 we used only the shortened ramp-up. Finally manageable, predictable toxicity show that it could be a good schedule for an outpatient setting.
Methods: We enrolled all pts with RRMM from a single institution after at least two lines of therapy including triple-class exposure to an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 antibody ; 13 pts had standard ramp-up (Gp1) over 5 days accorded to the guidelines ; 27 pts had received a shortened ramp-up (Gp2) over 4 days (Day(D)1: 0.06mg/kg, D2: 0.3 mg/kg, D4: 1.5 mg/Kg) with no pause between the 1st and the 2nd dose . Data was collected from medical records between september 2022 until 01/05/2024.
Results: Between September 2022 and May 2024 , 40 patients were included; median age was 68 (range 37-84); 16 pts (40 %) had >75 years; 14 pts (35 %) were males. Patients received a median of 4 of lines of previous therapy (range 2-8); 28,1% were refractory to last line of therapy; 9 pts (22.5%) had high-risk cytogenetics/FISH.
In the total population CRS was seen in 24 (60%) (all G1: except 2 G2, 1 G3), all resolved within 24 hours and was managed with acetaminophen alone (n=17); tocilizumab(toci) alone (n= 1) ; dexamethasone(dex) alone (n=3) or dex- toci (n=2).
CRS was observed by 9 pts (69,23 %) in the Gp 1 (77,8 % G1, 22,2% G2), 22,2 % at D2; 44,4% at D4; 33,3% at D6 vs 14 pts (51,85%) in the Gp 2 (78,5% G1, 14,2% G2, 7,1% G3) 78% at D3, this may be due to the premedication of 2nd dose with dex which probably avoids the CRS after D1; and 21,4% at D5. ICANS was infrequent (n=2 ; 5 %) all G1.
The overall response rate for evaluable patients (n=39) was 61.53%; (≥VGPR in 22; PR in 2; PD in 15). With a median follow-up of 11,7 months(m), the median PFS and OS were 12,14 m and 13,04 m respectively. The median of duration of response (mDOR) is 12,47 months. Efficacy were similar in the two groups. Most common reason for treatment discontinuation was PD (38,4%). 15 pts have died by 01/05/24.Most common cause of death was disease progression.
Infections were common, seen in 19 (48,7%) of pts, the majority were viral Infections or Pneumonia. IVIG was given in 19 pts (48,7%). There was no difference between the two groups.
Conclusions: In this study, results of adverse events (CRS, ICANS), ORR and PFS were similar to MajesTEC-1. The rates of CRS in Gp2 was low compared to the Gp1 and occured at D3 or less frequently at D5 i.e. one day less CRS. Moreover, shortened Ramp-up do not compromise pts outcome and toxicity. Since 02/23 we used only the shortened ramp-up. Finally manageable, predictable toxicity show that it could be a good schedule for an outpatient setting.